The main conclusions of many investigations about general requirements of plants for spectral composition of PAR are based on phylogenetic aspects of plant growth (Kleshnin et al., 1980; Geiger, 1994; et al). We think that these aspects are not the main criteria in choosing the spectral composition required for growing plants in controlled conditions. Our approach to this problem is based on plant and crop reaction under long duration growth with specific spectra and intensity. Only in this way can we determine correctly the role of light characteristics for developing crops.
Why does it happen? In this connection it will be useful
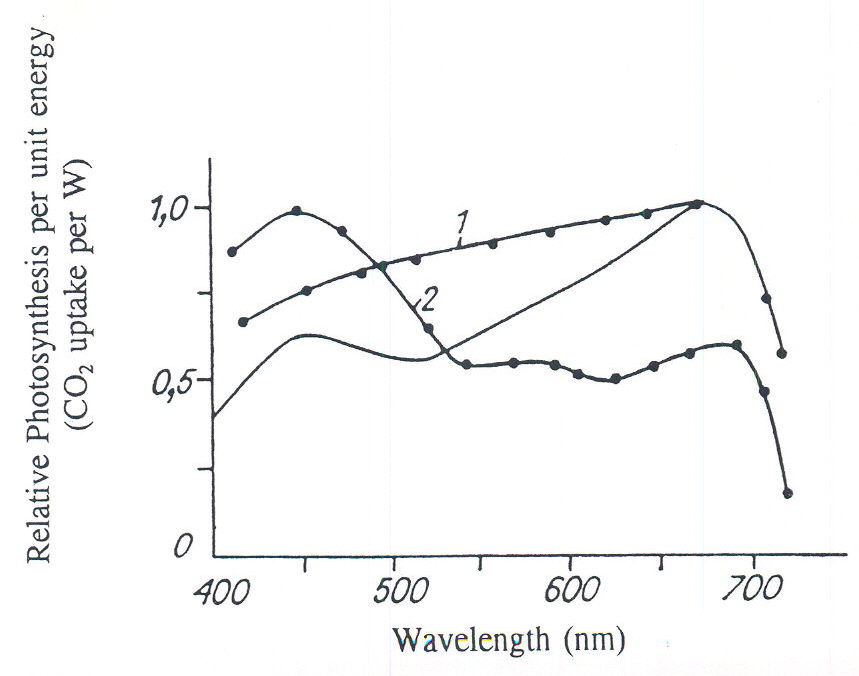
to examine the curve of the action spectrum of photosynthesis. This classical curve is formed under controlled influence of light that involves av 3-5 minutes irradiation with one specific spectral flux. The form of this curve is similar for green leaves of different species of plants. We’ve obtained different curves for spectral affectivity of green leaf photosynthesis (on the example of radish), when plants have had long duration adaptation (for some days) to lamps of different spectral composition and PAR intensity (Fig. 1, curves 1 and 2). The spectral of these lamps is shown in the Short Note by Prikupets and Tikhomirov in this publication. We feel the obtained differences have the following reasons. During short time intervals, only reactions of quick photoregulation are possible. These reactions affect only functional characteristics of the photosynthetic leaf system. In that time, structural changes do not occur and these reactions to light aren’t observed. The classical action spectrum of photosynthesis (Fig. 1, solid line) shows the universal characteristics of green leaves of different plant species. Plants have the same types of green pigments (chlorophyll), photosystems, reaction centers, pathways of energy migration to reaction centers and so on (Tikhomirov et al., 1987; 1991).
Fig. 1. The relative spectral efficiency of photosynthesis of green leaves of radish plants grown for 15 days under different PAR spectral lighting. Photosynthetic measurements for 400-500 mm were taken with plants grown under blue-light lamps, for 500-600nm under green light lamps, for 600-700nm under red-light lamps. 1) radiation intensity during growth of plants was 50 W m-2 PAR (radiation intensity for unsaturated photosynthesis). 2) radiation intensity during growth of plants was 50 W m-2 PAR (radiation intensity for unsaturated photosynthesis). 2) radiation intensity growth of plants was 200 W m-2 PAR (radiation intensity for saturated photosynthesis) (Tikhomirov et al., 1987;1991). Solid line is average value of relative spectral photosynthetic efficiency of green leaves according to McCree (1972), Inada (1976, 1977).
Therefore, usage of the curve for action spectrum of photosynthesis is not correct in light regulation under long-term stationary regimes, since certain reactions to spectrum and intensity of PAR aren’t taken into consideration. All spectral requirements obtained under short light influence tests have similar limitations.
What should be done? It’s necessary to be guided by data obtained under long-term influence of spectral and intensity characteristics on photosynthetic plant systems and even better on canopies of plants. These are the photosynthetic structures which ultimately form to produce the harvested yield. We’ve obtained some results which support this conclusion. These are some of the universal responses (Tikhomirov et al., 1991):
1) The time for maximum affectivity of photosynthesis of plant canopies appears earlier with red (600-700 nm) and later may shift to shorter wave length regions of PAR. This shift depends on specific plant reaction to spectrum of PAR;
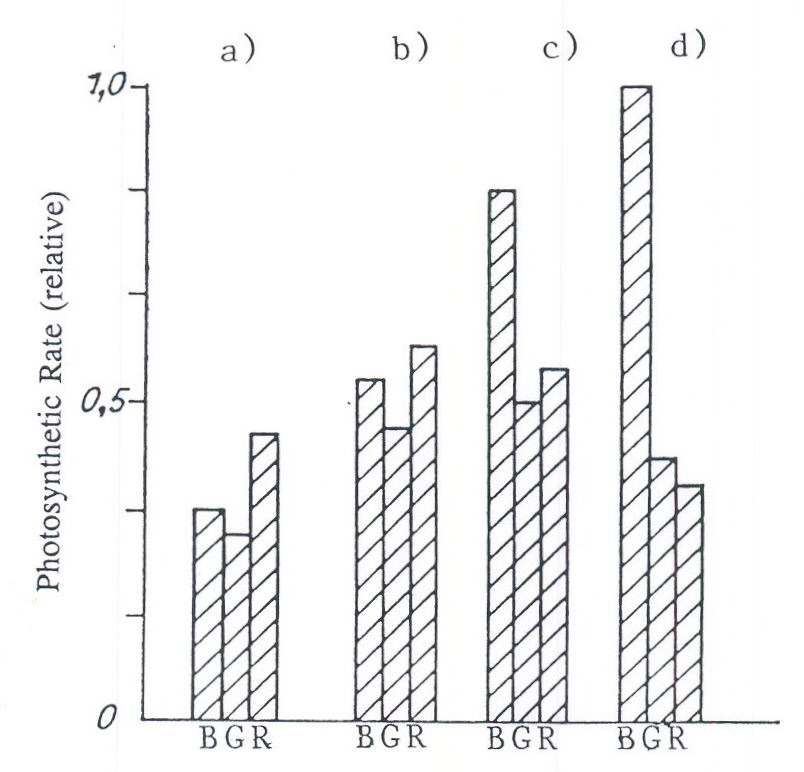
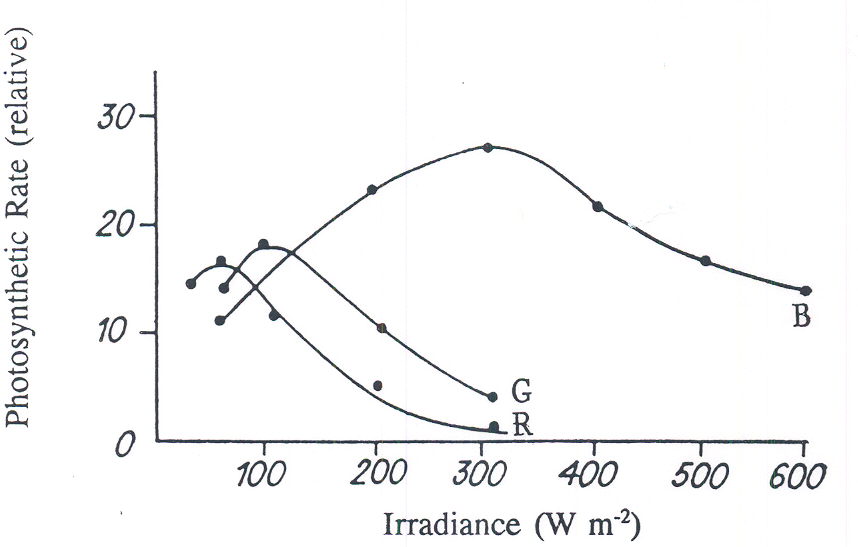
2) The relative effectiveness of blue rays increases and green and red rays decreases with higher levels of irradiation (Fig. 2 and 3);
3) Maximum photosynthesis of canopies is possible only under combinations of blue, green and red radiation. Any kind of combinations of two of these wavebands or with only one spectral region, always reduces productivity.
Fig. 2. The relative spectral efficiency of photosynthesis of cucumber leaves adapted to long duration growth with radiation of different energy and spectral composition. B = 400 – 500nm, G = 500 – 600nm, R = 600 – 700nm. a) 12 W m-2 (Ko, 1974); b) 24 W m-2 (Ko, 1982); c) 50 W m-2 (Tikhimirov et al., 1991); d) 100 W m-2 (Tikhomirov et al., 1991).
Fig. 3. The photosynthetic rate of 15d-old radish canopies with irradiance of differing spectral composition and intensities (B = 400-500nm, G = 500-600nm, R = 600-700nm). (Tikhomirov, et al., 1991).
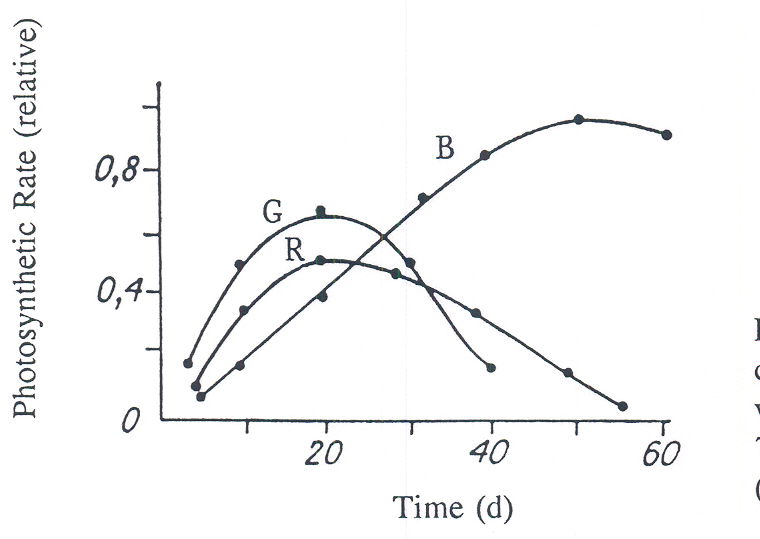
It is not necessary to provide light conditions for maximum photosynthesis of every plant leaf but to provide light conditions for optimal photosynthesis of the plant canopy. As a general rule over long periods, photosynthesis systems grow old very quickly. The effect of leaf age on photosynthic rate is shown in Figure 4 using cucumber.
Fig. 4. The photosynthetic raate of cucumber per canopy area for PAR irradiances of 100 W m-2 with 400-500nm (B), 500-600nm (G), or 600-700nm (R) during canopy development (Tikhimirov et al., 1991).
Optimal photosynthesis of plant leaves involves a harmonious relationship between spectrum and the intensity of PAR. Thus, plants “work” to obtain the maximum photosynthetic efficiency (but the photosynthesis is not maximum for each leaf) (Tooming, 1984).
There is a question. Is it necessary to prepare optimal light conditions for the photosynthesis of all leaves on the plant or not? What way is it determined? The correct decision on spectral composition of light depends very much on certain morphological characteristics of plants. There is a dependance upon the distribution of fruits along a stem (Tikhomirov, 1990). For example, cucumber has equal distribution of fruits along the stem. There every leaf supplies assimilate to its fruit. In this connection cucumber leaves at all layers must be provided with optimal light conditions. This requires a large portion of green rays in PAR (about 40%). Red rays in PAR (about 40%) provide high level of photosynthesis of upper leaves. Green rays penetrate into middle and lower leaves of plants. Blue rays have regulatory function, but its part in PAR is not very big (about 20%) (Tikhomirov, 1989).
We have another situation, where fruits of a plant concentrate in the upper part of the stem. Classical example is wheat. The ear of wheat is supplied with assimilates, primarily from the upper leaves. With this crop, PAR must have approximately 60-70% red rays (Tikhomirov, 1990). We’ve obtained data on specific reactions of plants for the spectral composition of PAR. It’s particularly important during plant development processes. According to this point of view plants may be divided into two groups (Tikhomirov et al., 1991):
1) The first group is characterized with restricted growth and development processes at definite ontogenetic phases if the PAR spectrum and intensity are not optimized (i.e., cucumber, sunflower);
2) The second group include plants capable of passing through all ontogeneticS phases and producing a harvest irrespective of the PAR spectrum and intensity provided (for example: tomatoes, wheat).
Wheat is capable of passing through all phases of ontogenesis regardless of any specific spectral irradiation. It’s correct for PAR range of 100-600 W m-2 and possibly even higher. Tomatoes have more restricted PAR range in comparison with wheat. With a PAR of 200 Wm-2 and higher, tomato productivity is lowered in red rays. PAR range for radish appeared to be more narrow. Even when red and green light is equal at a PAR of 200 Wm-2,plants of radish perish. Cucumbers appeared to be the most greatly influenced by the spectrum of PAR. For example in red wavelengths with less than PAR of 50 Wm-2, plants die.
We shouldn’t ignore these great differences in reaction of plants on spectrum and PAR intensity. All compromising decisions including introduction of universal spectrum of irradiation lead to partial loss of productivity.
Equal-energetic spectrum (“white” light) or a spectrum similar to curve of the action spectrum of photosynthesis have been proposed for use as a universal spectra for plants growing under lamp lighting. The first might be chosen because of consideration of phylogenesis of plants, the second – because of research familiar to you (McCree, 1972; Inada, 1976). Either of these options could be accepted as a temporary compromise for initial research.
I do not believe that we have to copy illumination of plants in natural conditions for use in controlled environment growing. For example there’s no need to grow some species of plants under alternative light dark periods. Our research showed that productivity of some plants (radish, wheat) can be increased under continuous irradiation (Tikhomirov et al., 1976; Lisovsky et al., 1987). Also, we should not strictly aspire to duplicating morphophysiological characteristics of field grown plants. Thus, for example, we achieved a very large radish productivity when we sharply changed its photomorphogenesis (Tikhomirov et al., 1976). This is true for increasing cucumber productivity too. However, if we accept this concept, we must know where and how we should deviate from natural conditions to increase productivity of plants grown in controlled environments. So I have the following suggestions:
1) As a temporary measure the draft guideline distributed by the organizing committee might be recommended for usage for plants grown in controlled environments;
2) Research work should be expanded to identify the spectrum of PAR radiation for each plant species which provides the maximum crop value.
If these suggestions are taken into consideration, my colleagues from Russia and I are ready to discuss a program of research and take part in its conduct.
REFERENCES
Geiger, D. 1994. Spectral composition of light and growing of plants in controlled environments. (Report at present Workshop.)
Inada, K. 1976. Action spectra for photosynthesis in higher plants. Plant and Cell Physiology, 17:355-365.
Kleshnin A.F. Moskow, 1954. A plant and light. (In Russian) Moscow Academy of Science, Moscow, USSR.
Lisovsky G.M., Sid-ko F.Y., Polonsky V.I., Tikhomirov A.A., ZoLOtukhin I.G. 1987. Light intensity and quality as factors determining plant stand formation and yield under controlled artificial illumination. Russian Plant Physiology. 34:636-643.
McCree K.J., The action spectrum, absorbance and quantum yield of photosynthesis in crop plants. Agric. Meteorology 9:191-216.
Tikhomirov A.A. 1990. Spectral efficiency of productivity process and photometric aspects of light culture of plants. (Doctoral dissertation in Biophysics). Krasnoyarsk, Institute of Biophysics.
Tikhomirov A.A. 1989. Photosynthesis of cucumber plants formed during radiation of different spectral composition of PAR. Ukrainian Journal Physiology and Biochemistry of Plants. 21:3-8.
Tikhomirov A.A., Lisovsky G.M., Sid-ko F.I. 1991. Spectral composition of light and plant productivity (In Russian). Nauka, Novosigirsk, USSR
Tikhomirov A.A., Zolotukhin I.G., Sid’ko F.Y. 1976. The influence of light conditions on the productivity of quality of radish crop. Russian Plant Physiology. 23:502-507.
Tikhomirov A.A., Zolotukhin I.G., Lisovsky G.M., Sid’ko F.Y. 1988. Specific responses in various plant species to the spectral composition of Photosynthetically active radiation under artificial illumination. Russian Plant Physiology. 34:774-785.
Tooming X.G., 1984. Ecological principles of maximal productivity. (In Russian) Gidrometedizdat, Leningrad, USSR.
Tikhomirov, A.A. 1994. Spectral composition of light and growing of plants in controlled environments, p 25-29. In: T.W.Tibbitts (ed.). International Lighting in Controlled Environments Workshop, NASA-CP-95-3309.
Copyright © March 1994 NASA [National Aeronautics and Space Administration].
All rights reserved.