INTRODUCTION
An important motivation for studying photomorphogenesis is to understand the relationships among plant photophysiology in canopies, canopy productivity, and agronomic yield. This understanding is essential to optimize lighting systems used for plant farming in controlled environments (CE) and for the design of genetically engineered crop strains with altered photoresponses. This article provides an overview of some basic principles of plant photomorphogenesis in canopies and discusses their implications for (1) scaling up information on plant photophysiology from individual plants in CE to whole canopies in the field, and (2), designing lighting conditions to increase plant productivity in CE used for agronomic purposes [e.g. space farming in CE Life-Support-Systems (Bugbee and Salisbury 1989)]. We concentrate on the visible (λ between 400 and 700 nm) and far red (FR; λ> 700 nm) spectral regions, since the ultraviolet (UV; 280 to 400 nm) is covered by other authors in this volume.
NEIGHBOR DETECTION IN PLANT COMMUNITIES
The spectral distribution of sunlight changes dramatically as the light beams interact with vegetation. Light is strongly scattered inside plant tissues, and leaf pigments absorb most of the UV and visible parts of the spectrum. In contrast, relatively few FR quanta are absorbed, and most of them exit plant organs in the form of scattered radiation. Therefore, within plant canopies the light climate is characterized by low levels of blue (B) and red (R) light (the visible wavelengths that are most absorbed by chlorphylls) and high levels of FR.
Changes in R:FR ratio are used by plants to monitor the proximity of neighboring individuals (for reviews, see Ballaré et al. 1992b, Sánchez et al. 1993, Ballaré 1994). R:FR sensing by phytochrome was originally proposed as a mechanism for the perception of leaf shading by seeds and plants occurring underneath vegetation canopies (Taylorson and Borthwick 1969). Thus, variations in R:FR caused by preferential absorption of R light by chlorphylls would shift the amount of phytochrome present as Pfr in plant tissues. This change in the amount of Pfr would provide a cellular signal that, being related to the degree of shading, could be used by plants in the understory to control developmental timing and morphogenesis. This idea has been supported by spectroradiometric studies in plant canopies (e.g. Kasperbauer 1971, Holmes and Smith 1977) and physiological experiments in CE (Taylorson and Borthwick 1969, Morgan and Smith 1978, Child and Smith 1987).
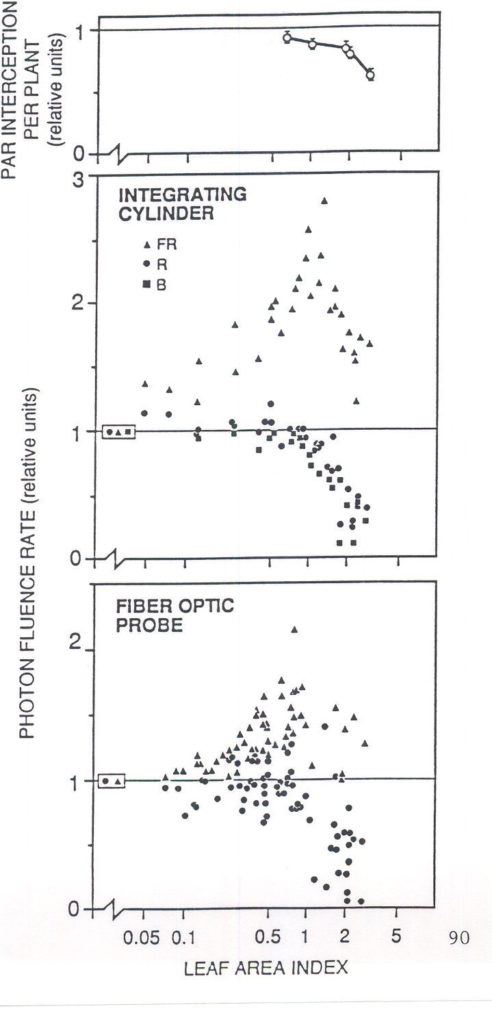
postulated that FR light, back-scattered by neighbors, may provide to each individual seedling an “early-warning signal” of impending competition, before the onset of severe shading among plants (Ballaré et al. 1987). Figure 1 shows how light scattering by plant tissues increases the fluence rate of FR received by vertically-oriented internodes as the leaf area index of a seedling canopy is increased, and how this spectral shift precedes variations in photosynthetically active radiation (PAR) at the leaf level. The ability of plants to remotely detect their neighbors using the R:FR spectral shift has now been demonstrated using a suite of experimental approaches, which involved manipulations of the light environment received by isolated plants growing under natural radiation (e.g. Ballaré et al., 1987, 1991a, Casal et al. 1987, Novoplansky et al. 1990), manipulations of the light environment in plant canopies (Casal et al. 1986, Ballaré et al. 1990), and the use of mutants deficient in R:FR sensing (Ballaré et al. 1992a, Casal and Kendrick 1993) .
Fig. 1. Effects of increasing the leaf area index (m2leaf area / m2soil area) in even-height canopies of dicotyledonous seedlings on light interception by leaves (top) and the light climate of the stems. The integrating cylinder collects sidelight received by the stem surface; the fiber optic probe collects light scattered within the stem tissue. All values are given relative to the measurements obtained for isolated plants or for leaf area index » 0 (boxed symbols). Abbreviations: B, blue; FR, far-red; PAR, photosynthetically active radiation; R, red. (From Ballaré 1994; original data in Ballaré et al. 1991b.).
Changes in photon fluence rate can also convey information about the proximity of neighbors in plant canopies. For plants growing underneath other vegetation, a change in the leaf area index of the canopy will cause variations in irradiance that plants may use as an input signal for the systems that control shade acclimation at different levels, from chloroplast physiology to whole-shoot allometry (e.g. Blackman and Wilson 1951, Björkman 1981). Moreover, changes in light fluence rate may also work as early proximity signals in even-height canopies of broadleaf seedlings (Ballaré et al. 1991a), because fluence rate sensed by vertically-oriented stems is more affected by changes in canopy density than the light climate of horizontal or diaphototropic leaves (Fig. 1).
Plants can “measure” fluence rate in two ways: (1) indirectly, by sensing changes in the availability of photosynthetic products (sugars), or (2) more directly, by sensing molecular signals closely related to the photoexcitation of the chloroplast photosystems or other photoreceptors (e.g. B-absorbing photoreceptors and phytochromes). Morphological responses to sucrose levels have been demonstrated (Montaldi 1969, Casal and Sánchez 1992) and changes in ATP and NADPH production (caused by variations of light intensity) may elicit changes in photosystem stoichiometry and organization, with consequences on photosynthetic capacity have been reported (Chow et al. 1990). Morphological responses to irradiance changes sensed by phytochrome (Ballaré et al. 1991a) and a B-absorbing photoreceptor (Britz 1990) have been extensively documented in studies with de-etiolated plants grown under high PAR. Experimental evidence supports the notion that plants growing in canopies use fluence rate signals perceived by these photoreceptors in the process of neighbor detection, and respond with morphological changes that presumably improve their light-harvesting ability in crowded populations (see below).
INFORMATION AND VEGETATIVE MORPHOLOGICAL DEVELOPMENT
Plants have evolved molecular mechanisms that use information about the canopy light environment, obtained through photoreceptors, to “decide” among alternative developmental programs. In this section we will briefly consider developmental photoresponses that involve changes in: (1) the rate of growth in height, and (2) the direction of vegetative spreading.
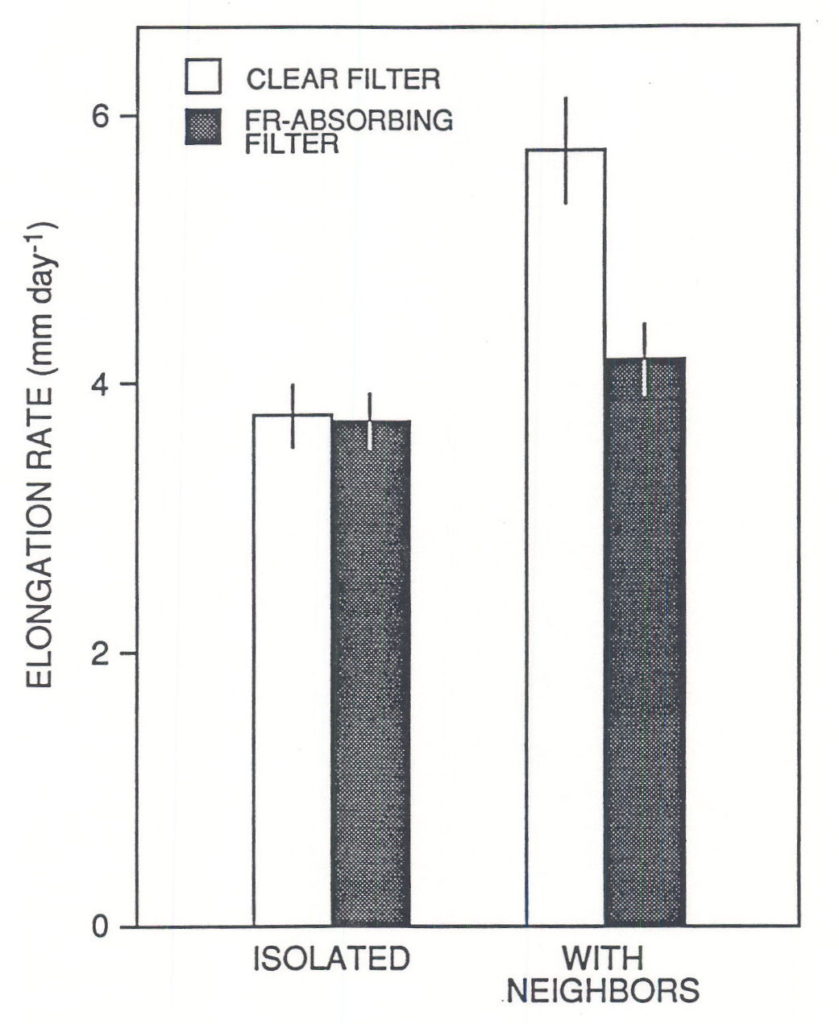
Reductions of R:FR promote stem elongation rate. This has been demonstrated for plants that received low levels of visible light (ca. £ 10 % of full sunlight, Kasperbauer 1971, Morgan and Smith 1978, Child and Smith 1987), and plants grown under natural radiation supplemented with FR provided by selectively-reflecting mirrors (Ballaré et al., 1987, 1991a). Manipulative experiments with even-height canopies of seedlings have shown that the reduction in R:FR of the scattered radiation that impinges laterally on the internodes (Fig. 1) can trigger an increase in elongation rate, even if most of the leaf area is exposed to full sunlight (Fig. 2; Ballaré et al. 1990). Although direct evidence is still lacking, most of the physiological data suggest that the decrease in fluence rate experienced by plant stems when the canopy begins to close (Fig. 1; leaf area index ³ 1) does elicit an increase in elongation rate before shading at leaf level becomes significant (Ballaré et al., 1991a). The increase in elongation rate triggered by R:FR and fluence-rate signals is almost certainly beneficial for the individual plant, because, in a rapidly growing canopy, a small difference in height would imply an inordinately large difference in PAR harvesting (e.g. Ballaré et al., 1988).
As they grow in a heterogeneous canopy, plants can acquire information about the spatial distribution of their neighbors using fluence rate and R:FR signals perceived by phytochromes and B-absorbing photoreceptors. Irradiance gradients elicit phototropic movements of plant leaves (Koller 1990) and stems (Iino 1990), which presumably increase the light harvesting capacity of plant shoots in horizontally patchy canopies. Novoplansky et al. (1990) suggested that seedlings of the plageotropic herb Portulaca oleracea use alterations in the R:FR ratio of the scattered canopy light to effectively avoid their neighbors. Their manipulative physiological experiments under natural sunlight have supported this hypothesis. Ballaré et al. (1992a) have shown that cucumber plants use the phytochrome system and a B-absorbing photoreceptor to remotely detect their neighbors and to elicit stem bending responses toward canopy gaps (Fig. 3).
Fig. 2. Elongation responses of Datura ferox first internodes when seedlings were placed in the center of an even-height canopy of leaf area index » 0.9 under natural radiation. During the experiment, which run for 3 days, the internodes were surrounded by annular cuvettes containing distilled water (clear filter) or a CuSO4 solution that absorbed FR radiation and maintained the R:FR ratio at ca. 1.1 (FR-absorbing filter). (Adapted from Ballaré et al. 1990.)
Fig. 3. Effects of the proximity of a green maize canopy and B-absorbing acetate filters (B barrier) on the orientation of the hypocotyls of WT and lh-mutant seedlings. Seedlings were grown in the field for 2 days at the center of a clear plot (isolated) or 8 cm to the south of the edge of a dense maize crop (canopy). Seedlings of the lh-mutant do not present phototropic responses to R:FR gradients, but display normal phototropism in response to B light. All the southward (i.e. “neighbor-avoiding”) bending induced by the nearby maize canopy can be abolished by eliminating the B light irradiance gradient created by the presence of the canopy (cf. Control vs. B barrier in panel C). Compared with lh seedlings, WT seedlings display more intense bending in response to the proximity of the maize canopy, and a significant proportion of this bending cannot be accounted for by phototropic responses to B light gradients (cf. Control vs. B barrier in panel D). (From Ballaré et al. 1992a).
Apart from being able to use light signals in the control of vegetative morphogenesis, plants appear to have evolved mechanisms to relay information about the canopy light environment into systems that control reproductive allocation. The potential agronomic significance of this aspect of plant photomorphogenesis has been discussed (Ballaré et al. 1992b; Sánchez et al. 1993), and will not be covered in this article.
CONSEQUENCES AT THE POPULATION LEVEL
Very little is known about the consequences of the photomorphogenic behavior of individual plants at the population-level (e.g. Schmitt and Wulff 1993, Ballaré 1994). A common belief is that most plant responses to proximity signals (teleologically called “shade-avoidance responses”) are selected through evolution because they confer an advantage to the individual plant, but that they would have normally a negative impact on canopy productivity or crop yield. On the basis of this idea, the need of eliminating these responses has been voiced by a number of authors. Two avenues have been proposed to accomplish this goal: (1) to artificially increase the R:FR ratio received by the canopy (in CE), and (2) to engineer photomorphogenically “blind” plant cultivars. In this section we will briefly discuss the likely implications of elongation and tropic photoresponses for whole-canopy productivity.
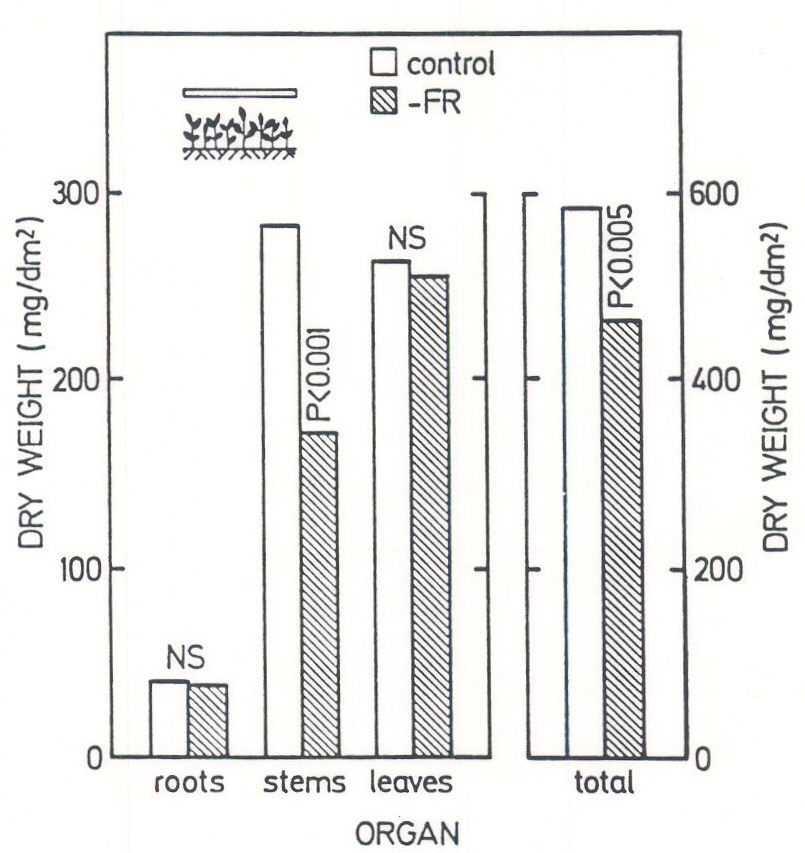
Exposure to low R:FR ratios normally results in plants having long internodes and low leaf-to-stem dry weight ratio (LSR) (e.g. Morgan and Smith 1978). One interpretation of the change in LSR is that the low R:FR triggers, through phytochrome, a re-distribution of C, away from the leaves and toward the stem. According to this view, stem growth responses to reduced R:FR may negatively affect canopy yield, by taking up C that would otherwise be allocated to leaves, the main light-harvesting organs. Most of the evidence obtained from experiments under relatively high irradiances is not consistent with this hypothesis. The most significant findings of these experiments are the following (Ballaré et al. 1991b). (1) The amount of C allocated to stem growth is, at least for young herbaceous plants, a relatively small percentage of the total C budget. (2) A localized reduction of R:FR at the stem level can increase internode elongation rate by a factor of two and dry mater accumulation in the stems by 40% without having any negative impact on leaf or root growth. In fact, total plant biomass can be increased by a localized R:FR treatment, presumably through a feedback control over photosynthesis. (3) If whole canopies are grown under extremely high R:FR ratios, which nearly eliminate elongation responses to neighbor proximity, stem dry matter accumulation is reduced, but without yielding any proportional increase in leaf or root growth (Fig. 4). In summary, the C saving benefits of abolishing stem growth responses to neighbor-proximity signals are likely to be very small or nil.
We have discussed in the preceding sections how sensing of radial R:FR gradients allows plants to monitor the spatial distribution of their immediate neighbors. This information, acquired through phytochrome, triggers phototropic responses that presumably optimize shoot geometry and spreading as a function of the spatial distribution of light gaps in the canopy. For instance, long-term experiments in the field have shown that wild-type cucumber plants are much more efficient at deploying leaf area into gaps than plants of an isogenic lh mutant that lack immunochemically-detectable phytochrome B. Shoot geometry and space occupation are mayor determinants of whole-plant C-assimilation (e.g. Küppers, 1994). Therefore, if we move up in scale one step, i.e. from single shoots to a shoot population, the inference would be that phototropic responses, triggered by R:FR gradients, are likely to be an important component of the mechanisms that allow the growing canopy to efficiently “fill-up” the aboveground space. In other words, at each point in time during canopy development, phototropic responses of individual shoots would increase light interception per unit of canopy leaf area.
Fig. 4. Effect of filtering out the FR wave-band from the light received by canopies of amaranth (Amaranthus quitensis) on canopy growth and dry matter allocation. FR was filtered using cuvettes containing CuSO4 solutions (see diagram upper left). This treatment increased the R:FR received at the top of the canopy from 1.1 (control; cuvettes filled with water) to 17.4 (-FR), and effectively reduced stem elongation. The bars indicate biomass present in the various organs after two weeks of treatment. The level of significance is indicated for each difference; NS = not significant (P>0.05). (Adapted from Ballaré et al 1991b).
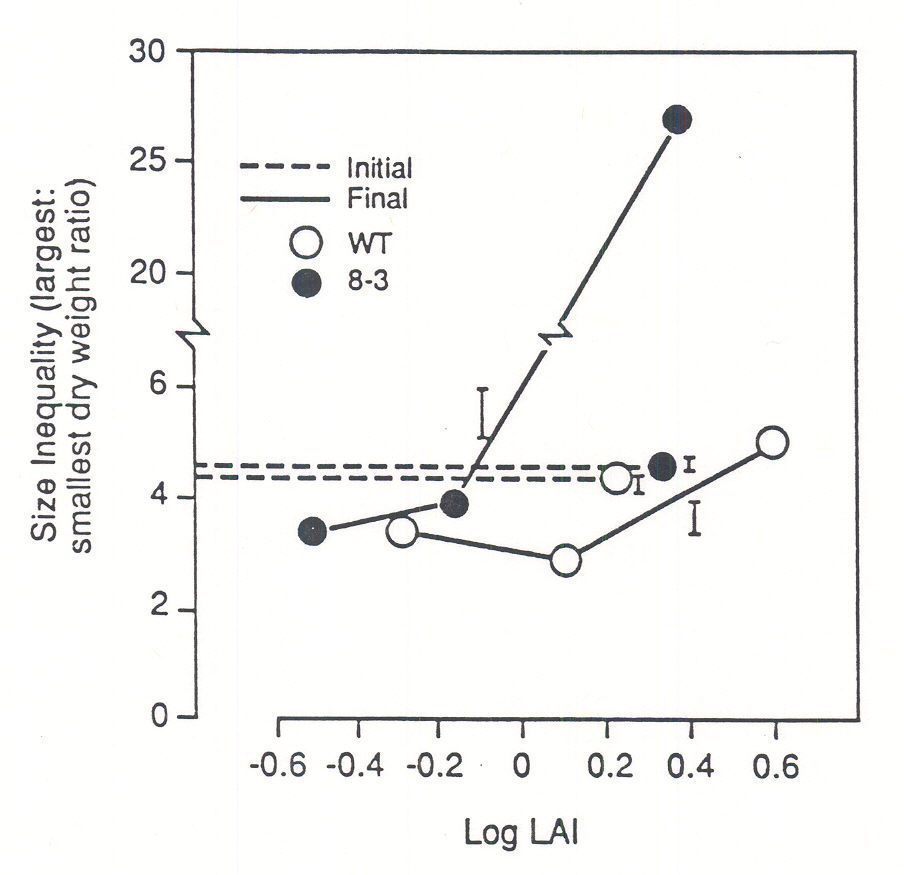
Most studies on canopy photomorphogenesis have focused on the average response of the components of a population, not on the variability among individual plants. Yet information on the latter is important if the goal is to predict the population-level consequences of plant photophysiology. The development of size (dry weight) inequalities among neighbors is one of the best characterized population responses to increased plant density (number of plants per unit area) (Harper 1977, Weiner 1985). Because reproductive output and size are often positively correlated within plant populations (e.g. Thompson et al. 1991), understanding the determinants of size variability is of fundamental importance for ecologists (Weiner 1985, Weiner et al. 1990) and growers (Harper 1977, Benjamin and Hardwick, 1986). Transgenic tobacco plants that express an oat phytochrome gene (phyA) under control of the CaMV35S promoter and display altered photophysiology have been recently used to test the role of light sensing in the genesis of size inequalities in plant populations (Ballaré et al. 1994). Compared with the isogenic wild-type, phyA-overexpressing plants showed dramatically reduced morphological responsivity to changes in the R:FR ratio of the incident light, and to the proximity of neighboring plants in spacing experiments. In transgenic canopies an increase in stand density caused the small plants of the population to be rapidly suppressed by their neighbors (Fig. 5). In wild-type canopies, plants responded to increased density with large morphological changes, and there appeared to be an inverse relationship between the magnitude of this morphological response and the ranking of the individual plant in the population size hierarchy (not shown). In these wild-type populations, size inequality increased only moderately with density within the time frame of the experiments (Fig. 5). These results suggest that, in crowded stands, the ability of individual plants to acquire information about their light environment via phytochrome plays a central role in driving architectural changes that, at the population level, delay the development of size differences between neighbors.
Fig. 5. Effects of increasing population density on the development of size inequalities among neighbors in monocultures of WT and 8-3 transgenic tobacco plants. Bars indicate +1 SE; n=5 (final) or n=15 (initial) replicate canopies. Dry weights were measured after 30 days of growth; data are plotted against the leaf area index (LAI) estimated for the 15th day. Initial inequality was within + 15 % of the plotted average (dashed line) in all density treatments. (From Ballaré et al. 1994).
In summary, although very little is still known from studies under realistic levels of PAR, the evidence discussed in this section suggest that, contrary to the ideas currently on fashion, interfering with the normal traffic of light signals between neighboring plants (e.g. by using extremely high R:FR ratios), or with the plants’ information-acquiring systems (e.g. by breeding photomorphogenically “blind” genotypes), is unlikely to result in an increase of canopy net primary productivity. The impact of such manipulations on harvestable yield is difficult to predict, mainly because of uncertainties regarding photomorphogenic controls of developmental timing (e.g. Mondal et al. 1986) and reproductive allocation (e.g. Heindl and Brun 1983). However, to the extent that size structuring compromises yield and yield uniformity, the available data suggest that elimination of plant photomorphogenic responses in canopies will result in reduced agronomic productivity.
IMPLICATIONS FOR LIGHTING IN CONTROLLED ENVIRONMENTS
Light conditions differ between controlled and natural environments in many regards, including: daily time course of irradiance changes, spatial distribution of the light field, total irradiance, and spectral balance. In this section we concentrate on the latter two aspects (total irradiance and spectral distribution). We will use some of the concepts developed earlier in this article to discuss how the use of unnatural irradiances and spectral distributions may affect (1) whole-canopy growth, and (2), the likelihood that results obtained in the CE may be properly extrapolated to the field situation.
Low Light Levels
Lighting fixtures in most CE, particularly in old designs, provide PAR irradiances that are between one twentieth to one half of the peak PAR in a clear summer day. This low PAR of course will limit canopy growth by limiting photosynthetic rates (e.g. Geiger, this volume). But in addition to the growth limitation, several aspects of plant morphogenesis are be altered by the use of low irradiances (e.g. Blackman and Wilson 1951). Of particular importance within the context of this article is the interaction between low PAR levels and proximity responses elicited by light signals. In the foregoing sections we have discussed evidence that changes in fluence rate may signal encroaching vegetation to plants growing in sparse canopies. Morphological responses to fluence rate, although readily observable under high light conditions (e.g. Ballaré et al. 1991a) have not been consistently detected in CE studies under low PAR (e.g. Child and Smith 1987). Of course there are many possible explanations for the differences between CE and field studies, and a complete treatment of this subject is far beyond the scope of this article. But, in principle at least, there are good reasons to suspect that the use of low background light levels in CE is in itself a major complicating factor in studies of photomorphogenic responses to total irradiance. In the same vein, the use of low PAR levels in CE might contribute to artificially inflate the opportunity cost of stem growth responses to low R:FR ratios. Thus, plants growing in the field might be able to compensate the increased C demand of rapidly elongating internodes with a slight increase in leaf photosynthesis, whereas plants that are already limited by light may be more likely to rely on re-distribution of their short supply of carbohydrates. Finally, under extremely low PAR levels, the extent of the response to R:FR may be affected, presumably as a consequence of assimilate limitations (Smith and Hayward 1985). Low light levels are also likely to accentuate the development of size hierarchies within the population (i.e. increase the coefficient of variation of dry weight per plant) (Schmitt et al. 1986), with potential negative consequences for yield and yield uniformity.
High R:FR Ratios
Fluorescent tubes and high pressure sodium vapor lamps are popular PAR sources in CE, and both provide R:FR ratios several times higher than sunlight. Due to the spectral properties of Pr and Pfr, changes in R:FR above ca. 1.5 do not cause a proportional change in the phytochrome photoequilibrium (Smith and Holmes 1977). Therefore, the R:FR-based neighbor detection mechanism is likely to be distorted or disabled when canopies are grown under extremely high R:FR ratios. Some experimental evidence for this idea has been provided by studies with amaranth, in which CuSO4 filters were used to increase the R:FR ratio of the light received by the canopies and reduce stem elongation. These studies have shown that very high R:FR ratios result in decreased (rather than increased) canopy net productivity. It is not clear whether this decrease is caused by (1) the elimination of an active sink of assimilates (i.e. the growing internodes), a change in the pattern of light penetration through the canopy (see below), or a combination of the two. In any case, these results appear to directly contradict the notion that canopy growth at high densities is limited by the diversion of photosynthate to “shade-avoidance” responses. Of course, the use of artificially high R:FR ratios may be a convenient way to obtain short-statured plants in CE, which may be desirable for many crops grown for horticultural or ornamental purposes (McMahon and Kelly 1990, Rajapakse and Kelly 1992).
Another predictable consequence of the use of extremely high R:FR ratios in CE is the elimination of phototropic responses triggered through phytochrome. Since these responses may play a role in the dynamics of gap-filling by the canopy, it is suggested that the increase in light interception over time (and therefore canopy growth) will be slowed under very high R:FR. Of course, the extent of this retardation would depend upon (1) the quantitative importance of phototropic responses in gap-filling by the shoot population, and (2), the extent to which phytochrome and B-absorbing photoreceptors play redundant roles in controlling phototropic responses in canopies.
Finally, very high R:FR, which disable the phytochrome-mediated mechanism of neighbor etection, will almost certainly result in increased size structuring in dense plant populations. From a plant grower stand-point the establishment of a strong size hierarchy in the population might have two negative consequences: reduced total yield at high densities and reduced yield uniformity.
ACKNOWLEDGEMENTS
Some of concepts stemmed from work supported by the Consejo Nacional de Investigaciones Científicas y Técnicas, the Antorchas Foundation (Argentina), and the Dept. of Forest Science, Oregon State University (USA). This support is gratefully acknowledged.
REFERENCES
Ballaré, C. L. 1994. Light gaps. Sensing the light opportunities in highly-dynamic canopy environments. p. 73-110. In: M.M. Caldwell and R.W. Pearcy (eds.). Exploitation of environmental heterogeneity by plants. Academic Press, San Diego, CA.
Ballaré, C. L., R. A. Sánchez, A. L. Scopel, J. J. Casal and C. M. Ghersa. 1987. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ. 10:551-557.
Ballaré, C. L., R. A. Sánchez, A. L. Scopel and C. M. Ghersa. 1988. Morphological responses of Datura ferox L. seedlings to the presence of neighbors. Their relationships with canopy microclimate. Oecologia 76:288-293.
Ballaré, C. L., A. L. Scopel, E. T. Jordan, and R. D. Vierstra. 1994. Signaling among neighboring plants and the development of size inequalities in plant populations. Proc. Natl. Acad. Aci. (In press).
Ballaré, C. L., A. L. Scopel, S. R. Radosevich and R. E. Kendrick. 1992a. Phytochrome-mediated phototropism in de-etiolated seedlings: Occurrence and ecological significance. Plant Physiol. 100:170-177.
Ballaré, C. L., A. L. Scopel and R. A. Sánchez. 1990. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247:329-332.
Ballaré, C. L., A.L. Scopel, R.A. Sánchez and S. R. Radosevich. 1992b. Photomorphogenic processes in the agricultural environment. Photochem. Photobiol. 56:777-788.
Ballaré, C. L., A. L. Scopel and R. A. Sánchez. 1991a. Photocontrol of stem elongation in plant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant Cell Environ. 14:57-65.
Ballaré, C. L., A. L. Scopel and R. A. Sánchez. 1991b. On the opportunity cost of the photosynthate invested in stem elongation reactions mediated by phytochrome. Oecologia 86:561-567.
Benjamin, L. R. and R. C. Hardwick. 1986. Sources of variation and measures of variability in even-aged stands of plants. Ann. Bot. 58:757-778.
Björkman, O. 1981. Responses to different quantum flux densities, p. 57-107. In: O.L. Lange, P.S. Nobel, C.B. Osmond, and H. Ziegler (eds.). Physiological plant ecology I. Responses to the physical environment. Vol. NS 12A of Encyclopedia of plant physiology. Springer Verlag, Berlin.
Blackman, G. E. and G. L. Wilson. 1951. Physiological and ecological studies in the analysis of plant environment. VII. An analysis of the differential effects of light intensity on the net assimilation rate, leaf-area ratio, and relative growth rate of different species. Ann. Bot. 15:374-408.
Britz, S. J. 1990. Photoregulation of root:shoot ratio in soybean seedlings. Photochem. Photobiol. 52:151-159.
Bugbee, B. G. and F. B. Salisbury. 1989. Current and potential productivity of wheat for a controlled environment life support system. Adv. Space Res. 9:(8)5-(8)15.
Casal, J. J. and R. Kendrick. 1993. Impaired phytochrome-mediated shade-avoidance responses in the aurea mutant of tomato. Plant Cell Environ. 16:703-710.
Casal, J. J. and R. A. Sánchez. 1992. Physiological relationships between phytochrome effects on internode extension growth and dry matter accumulation in light-grown mustard. Photochem. Photobiol. 56:571-577.
Casal, J. J., R. A. Sánchez, and V. A. Deregibus. 1986. Effects of plant density on tillering: the involvement of the R/FR and the proportion of radiation intercepted per plant. Expt. Environm. Bot. 26:365-371.
Casal, J. J., R. A. Sánchez, and V. A. Deregibus. 1987. Tillering responses of Lolium multiflorum plants to changes of red/far-red ratios typical of sparse canopies. J. Expt. Bot. 38:1432-1439.
Child, R. and H. Smith. 1987. Phytochrome action in light-grown mustard: kinetics, fluence-rate compensation and ecological significance. Planta 172:219-229.
Chow, W. S., D. J. Goodchild, C. Miller and J. M. Anderson. 1990. The influence of high levels of brief or prolonged supplementary far-red illumination during growth on the photosynthetic characteristics, composition and morphology of Pisum sativum chloroplast. Plant Cell Environ. 13:135-145.
Harper, J. L. 1977. Population biology of plants. Academic Press. London.
Heindl, J. C. and W. A. Brun. 1983. Light and shade effects on abscission and 14C-photoassimilate partitioning among reproductive structures in soybean. Plant Physiol. 73:434-439.
Holmes, M. G. and H. Smith. 1977. The function of phytochrome in the natural environment. II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochem. Photobiol. 25:239-245.
Iino, M. 1990. Phototropism: mechanisms and ecological implications. Plant Cell Environ. 13:633-650.
Kasperbauer, M. J. 1971. Spectral distribution of light in a tobacco canopy and effects of end-of-day light quality on growth and development. Plant Physiol. 47:775-778.
Koller, D. 1990. Light-driven leaf movements. Plant Cell Environ. 13:615-632.
Küppers, M. 1994. Canopy gaps: Light interception and economic space filling–A matter of whole-plant allocation. p. 111-144. In: M.M. Caldwell and R.W. Pearcy (eds.). Exploitation of environmental heterogeneity by plants. Academic Press, San Diego, CA.
McMahon, M. J. and J. W. Kelly. 1990. Influence of spectral filters on height, leaf chlorophyll, and flowering of Rosa x hybrida “Meirutral”. J. Environ. Hort. 8:209-211.
Mondal, M. F., J. L. Brewster, G. E. L. Morris and H. A. Butler. 1986. Bulb development in onion (Allium cepa L.) III. Effects of the size of adjacent plants, shading by neutral and leaf filters, irrigation and nitrogen regime and the relationship between red:far-red spectral ratio in the canopy and leaf area index. Ann. Bot. 58:207-219.
Montaldi, E. R. 1969. Gibberellin-sugar interaction regulating the growth habit of Bermudagrass (Cynodon dactylon (L.) Pers.). Experientia 25:91-92.
Morgan, D. C. and H. Smith. 1978. The relationship between phytochrome photoequilibrium and development in light grown Chenopodium album L. Planta 142:187-193.
Novoplansky, A., D. Cohen and T. Sachs. 1990. How portulaca seedlings avoid their neighbors. Oecologia 82:490-493.
Rajapakse, N. C. and J. W. Kelly. 1992. Regulation of chrysanthemum growth by spectral filters. J. Amer. Soc. Hort. Sci. 117:481-485.
Sánchez, R. A., J. J. Casal, C. L. Ballaré, and A. L. Scopel. 1993. Plant responses to canopy density mediated by photomorphogenic processes. p. 779-786. In: D.R. Buxton (ed.). International Crop Science I. Crop Science Society of America, Madison, WI.
Schmitt, J. and R. D. Wulff. 1993. Light spectral quality, phytochrome and plant competition. Trends Ecol. Evol. 8:46-51.
Schmitt, J., D. W. Ehrhardt and M. Cheo. 1986. Light-dependent dominance and suppression in experimental radish populations. Ecology 67:1502-1507.
Smith, H. and P. Hayward. 1985. Fluence rate compensation of the perception of red:far-red ratio by phytochrome in light-grown seedlings. Photochem. Photobiol. 42:685-688.
Smith, H. and M. G. Holmes. 1977. The function of phytochrome in the natural environment -III. Measurement and calculation of phytochrome photoequilibria. Photochem. Photobiol. 25:547-550.
Taylorson R. B. and H. A. Borthwick. 1969. Light filtration by foliar canopies: Significance for light-controlled weed seed germination. Weed Sci. 17:48-51.
Thompson, B. K., J. Weiner and S. I. Warwick. 1991. Size-dependent reproductive output in agricultural weeds. Can. J. Bot. 69:442-446.
Weiner, J. 1985. Size hierarchies in experimental populations of annual plants. Ecology 66:743-752.
Weiner, J., E. B. Mallory and C. Kennedy. 1990. Growth and variability of crowded and uncrowded populations of dwarf marigolds (Tagetes patula). Ann. Bot. 65:513-524.
Ballaré, C.L., and A.L. Scopel. 1994. Plant photomorphogenesis and canopy growth, p 89-102. In: T.W.Tibbitts (ed.). International Lighting in Controlled Environments Workshop, NASA-CP-95-3309.
Copyright © March 1994 NASA [National Aeronautics and Space Administration].
All rights reserved.