INTRODUCTION
Attempts to use artificially lit cabinets to grow plants identical to those growing in sunlight have provided compelling evidence of the importance of light quality for plant growth. Changing the balance of red (R) to far-red (FR) radiation, but with a fixed photosynthetic input can shift the phytochrome photoequilibrium in a plant and generate large differences in plant growth. With FR enrichment the plants elongate, and may produce more leaf area and dry matter (see Smith, 1994 these proceedings). Similar morphogenic responses are also obtained when light quality is altered only briefly (15-30 min) at the end-of-the-day (Ballare 1994; these proceedings). Conversely, for plants grown in natural conditions the response of plant form to selective spectral filtering has again shown that red and far-red wavebands are important as found by Kasperbauer and coworkers (Kasperbauer, 1992). Also, where photosynthetic photon flux densities (PPFD) of sunlight have been held constant, the removal of far-red alone alters plant growth (Mortensen and Stromme 1987; McMahon et al., 1991). As shown in Table 1 for chrysanthemum, with FR depletion plants grown in sunlight are small, more branched and darker green. Here we examine the implications for plant growth and flowering when the far-red composition of incident radiation in plant growth chambers is manipulated.
TABLE 1. Influence of filtered sunlight on growth and flowering of chrysanthemum cv Yellow Mandalay in long days of summer. Adapted from McMahon et al. (1991).
| Filter | R:FR | Pfr:Ptot | Plant Height
(cm) |
Leaf Chlorophyll
(g cm-2) |
Leaf Number | Visible Flowering (days) |
| Red
Blue Far-red Control |
1.16
0.99 3.3 1.16 |
.71
.66 .79 .71 |
30.3**
29.3 16.9** 28.6 |
36.7
39.5 55.4** 39.8 |
22
21.6 17.1** 21.5 |
>52
>52 46-49 >52 |
Significant differences; * p = 0.05 or ** p = 0.01 vs control
- R:FR ratio 655-665 nm vs 725-735 nm
- Pfr:Ptot calculated over 350 to 850 nm
FAR-RED ENRICHMENT AND PLANT GROWTH IN ARTIFICIALLY LIT CHAMBERS
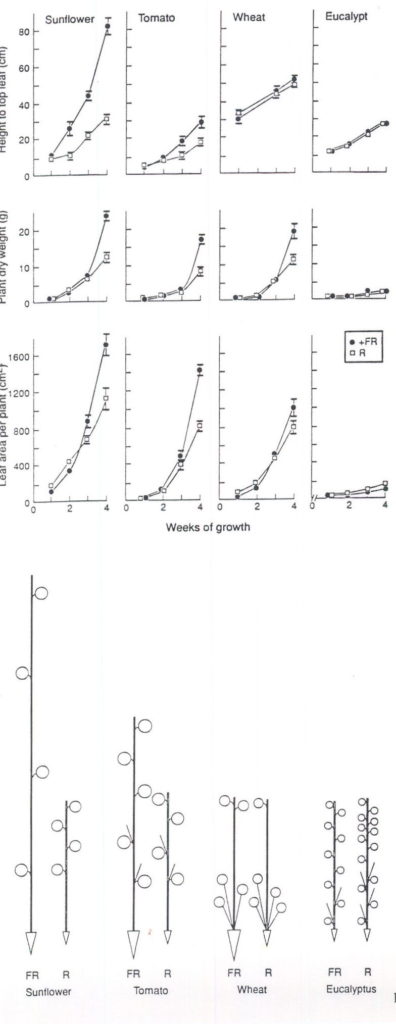
As with spectral filtering of sunlight, differences in the R:FR ratio of light in growth chambers can lead to large effects on growth (Figs 1, 2). Here PPFD was held constant across the two chambers (560 mols m-2s-1) and there were no major thermal differences, leaf temperatures across the chambers being within 1 to 2° C. Thus, the greater stem elongation with mixed metal halide/quartz halogen versus fluorescent lamps (Fig. 1) can be most simply explained as a phytochrome mediated response to FR enrichment. This FR-induced change was not driven by photosynthesis as stem elongation increased rapidly especially in sunflower (< 1 week, Fig. 1) and preceded by 1-2 weeks any increase in dry matter accumulation and leaf area production. To reiterate, there was firstly a change in plant form (Fig. 1), then later a change in dry weight indicating an initial photomorphogenically-driven increase in leaf area with subsequent photosynthetic increase, a conclusion suggested by Smith and coworkers from their studies over the last decade (see Smith 1994). On the other hand, photosynthetic capacity and/or leaf assimilate export could respond directly to FR enrichment. Chow, Anderson and coworkers have reported many FR effects on photosynthetic light harvesting pigment components particularly of young pea seedlings and these responses can result in slightly increased CO2 exchange rates per unit leaf area (Chow et al., 1990).
Surprisingly large effects on dry matter allocation to roots were observed as a consequence of FR enrichment (see Figure 2). Root growth has not always been measured in these types of experiment (e.g. Tibbitts et al., 1983) but there could also be a trivial explanation for the data summarized in Figure 2. Greater dry matter allocation to the roots was evident only at the last ( week 4) harvest. For tomato, for example, the root:shoot ratio doubled to 0.53 over the last week of growth in FR-enriched conditions whereas in the R-rich cabinet it remained at ca 0.3. However, this dry matter reallocation occurred when total dry matter was also increasing exponentially. Thus, the rapid increase in leaf assimilate supply may have temporarily exceeded stem demand leading to a shunting of assimilate to the roots and a transient shift in the root:shoot dry weight balance in FR-enriched conditions. Further studies are needed of responses of roots to FR-enriched conditions especially since our findings with tomato are the opposite of those noted earlier by Kasperbauer (1992) where only end-of-day light quality was altered.
Although sunflower and tomato grew optimally in FR-enriched conditions (Fig. 2) wheat was rather insensitive (Figs. 1,2) as also found for wheat by Tibbitts et al. (1983). The slower growth of the eucalypt (Fig. 1) may have masked positive responses. However, there was a significant reversal of response compared to other species in that FR-enrichment led to the formation of fewer leaves (61.2_3.5 vs 91.7_10.0) and branches (7.8_0.8 vs 13.2_1.5). This data for eucalypt requires confirmation but large differences in sensitivity to FR between species have been reported previously (Tibbitts et al., 1983 and see references therein).
Fig. 1. Growth in height, plant dry weight and leaf area of 4 plant species over 4 weeks of exposure to FR-rich (l ) or R-rich (o ) lamps. Irradiance 560 mol m-2s-1 of photosynthetically active radiation (PAR). The ratios of R:FR (660:730 nm) were FR=1.53 R=5.08. Daylength was 12-h with a day:night temperature of 21:16° C.
Fig. 2. Relative proportion of dryweight (R value set at 1) in root (Ñ ) stem (length of uprights) and leaf (circles x 2) after 4 weeks growth in a FR- or R-rich cabinet. Conditions as in Fig. 1.
FLOWERING AND FAR-RED ENRICHMENT
Potential for change in time to flower with FR enrichment is one morphogenic response that has received little attention in the designing of lamp types for plant growth chambers. For short-day plants, red rather than far-red rich conditions favour flowering (see Salisbury, 1965) but in most published reports there have generally been confounding effects of daylength change and photosynthetic input. With equalization of photosynthetic inputs as in the study of McMahon et al. (1991) with the short-day plant chrysanthemum, there was actually a slight enhancement of flowering time for plants grown in long days with removal of far-red (see Table 1). However, the response to the presence or absence of far-red may also vary with the time of the day. For the short-day plant Pharbitis nil a FR interruption of as little as 90 min during continuous light can promote or inhibit flowering depending on its timing (Heide et al., 1986). This FR response cycles with a period of about 12-h of a semidian rhythm (see Table 1).
By contrast with short-day plants, promotion of flowering by FR enrichment can be expected in long-day plants (see Vince-Prue, 1975; Deitzer, 1984). However, there are also very few comparisons of effects of luminaries on flowering of long-day plants when photosynthetic input has been fixed. In the studies of Tibbitts et al. (1983) mustard in 16-h long days reached anthesis about 2 to 3 d earlier (in a total of 25 to 29 d) when it was exposed to FR-rich lamps. Wheat was unaffected reaching anthesis at 57 d. However, an almost halving of days to flower (40 to 24 d) and of leaf number at flowering was found by Bagnall (1993) for the fca mutant of Arabidopsis (Landsberg strain) exposed to long days and ratios of R:FR ranging from 5.8 to 1.0.
The importance of FR-mediated effects of phytochrome on flowering in long-day plants is shown clearly by recent studies of Bagnall and coworkers (1994). They found that transgenic Arabidopsis plants constitutively overexpressing the far-red sensing phytochrome A gene flowered very early relative to the isogenic wild type (28 vs 64 d). Conversely, a mutant lacking phytochrome A is late to flower in FR-enriched conditions (Johnson et al., 1994) involving low PPFD (10 mol m-2s-1) FR-rich tungsten daylength extensions.
A PHOTOSYNTHETIC ROLE IN FLOWERING
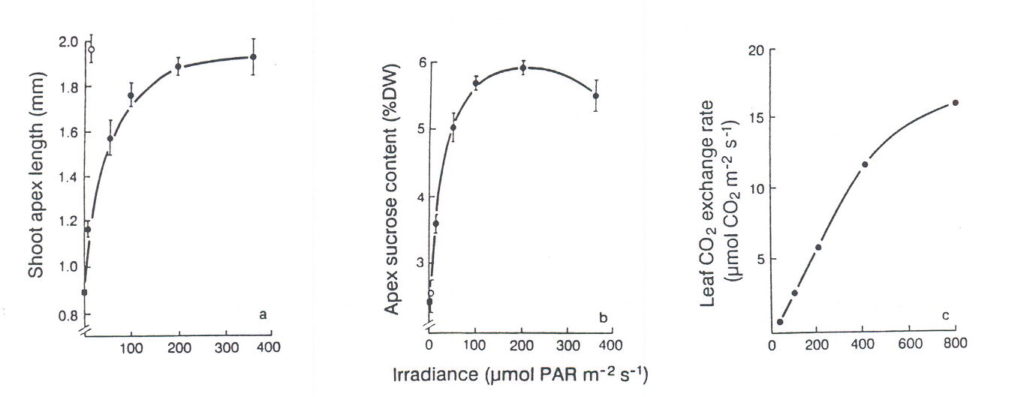
Although FR plays a central role in determining flowering time, photosynthetic input also influences expression of the long day response. Lolium temulentum, for example, flowers in response to a single long day and shows enhanced flowering the greater the photosynthetic irradiance. However, Lolium remains vegetative in short days independent of the irradiance of sunlight up to 1200 mol m-2s-1 (King and Evans, 1991). Photosynthesis alone is insufficient for flowering whereas a single non-photosynthetic long day given as a 16-h incandescent low-irradiance daylength extension is sufficient (Fig. 3) and less effective is an extension using a fluorescent, FR-deficient lamp. For either lamp, in association with a long day, increasing their photosynthetic contribution gives parallel and linear increases in flowering response (Fig. 3). There is no evidence here of interaction between light quality – the phytochrome-mediated, response – and the photosynthetic response to this long day. Thus, photosynthesis in Lolium must be considered as beneficial but not sufficient for flowering. On the other hand, a more direct photosynthetic effect is evident in another long day plant, Sinapis alba. Its requirement for a single FR-rich long daylength extension can be bypassed by increasing photosynthesis for more than three short days although this effect involves four times the photosynthetic irradiance applied during a single photoinductive long day (Bodson et al., 1977).
Since phytochrome and the photosynthetic pigments can act in concert to promote flowering of long-day plants, then, with increasing irradiance the photosynthetic contribution to flowering will range from nothing to apparently over-riding control by photosynthesis. As a consequence, action spectra could range from dominance by red wavelengths to a balance in the contribution by red and far-red and to the classic dominance by far-red wavelengths. The literature contains illustrations of all these combinations of wavelength and response of flowering to red and far-red. (Deitzer, 1984; Carr-Smith et al. 1989) and, clearly, some detailed reexamination of wavelength and irradiance interactions is required.
Fig. 3. Dependence on irradiance from fluorescent lamps during a single 16-h daylength extension of (a) flowering response in terms of shoot apex length after 3 weeks and (b) apex sucrose content at the end of the 16-h extension. Short day (n ) and a low PPFD incandescent 16-h extension (o) are also included. Leaf CO2 exchange (c) was determined during the main photoperiod. From King and Evans (1991).
OVERVIEW
As a broad generalization, far-red rich lamps are beneficial and sometimes essential, for plant growth and flowering in artificially lit chambers. Thus fluorescent and sodium lamps, being FR deficient may cause stunting and poor flowering. Brief end-of-day FR exposure may alleviate some of the stunting of growth but will probably have complex effects on flowering. A more beneficial approach appears to be continued FR enrichment over the whole photoperiod. A further complexity is that the need for FR input may vary cyclically over the day.
REFERENCES
Bagnall, D.J. 1993. Light quality and vernalization interact in controlling late flowering in Arabidopsis ecotypes and mutants. Annal. Bot. 71: 5-83.
Ballare, C.L. and A. L. Scopel. 1994. Plant Photomorphogenesis and Canopy Growth. (This proceedings)
Bodson, M., R.W. King, L.T. Evans and G. Bernier. 1977. The role of photosynthesis in flowering of the long-day plant Sinapis alba.
Carr-Smith, H.D., C.B. Johnson and B. Thomas. 1989. Action spectrum for the effect of day-extensions on flowering and apex elongation in green, light-grown wheat (Triticum aestivum L.) Planta 179:428-432.
Chow-, W.S., D.J. Goodchild, C., Miller and J.M., Anderson. 1990. The influence of high levels of brief or prolonged supplementary far-red illumination during growth on the photosynthetic characteristics, composition and morphology of Pisum sativum chloroplasts. Plant, Cell, Environ. 13:135-145.
Deitzer, G.F. 1984. Photoperiodic induction in long-day plants. p.51-68. In: D. Vince-Prue, B. Thomas and K.E. Cockshull (eds.). Light and the flowering process. Academic Press, London.
Heide, O.M., R.W. King and L.T. Evans. 1986. A semidian rhythm in the flowering response of Pharbitis nil to far-red light I. Phasing in relation to the light-off signal. Plant Physiol. 80:1020-1024.
Johnson, E., N.P. Harberd and G.C. Whitelam. 1994. Photoresponses of light grown PHYA mutants of Arabidopsis. Phytochrome A is required for the perception of daylength extensions. Plant Physiol. In Press.
Kasperbauer, M.J. 1992. Phytochrome regulation of morphogenesis in green plants: from the Beltsville spectrograph to coloured mulch in the field. Photochem. and Photobiol. 56:823-832.
King, R.W. and L.T. Evans. 1991. Shoot apex sugars in relation to long-day reduction of flowering in Lolium temulentum. Aust. J. Plant Physiol. 18:121-135.
McMahon, M.J., J.W., Kelly, D.R., Decotean, R.E., Young and R.K. Pollock. 1991. Growth of Dendranthemum x grandiflora (Ramat.) Kitamura under various spectral filters. J. Amer. Soc. Hort. Sci. 116:950-954.
Mortensen, L.M. and E. Strømme. 1987. Effects of light quality on some greenhouse crops. Scientia Hortic. 33:27-36.
Smith, H. 1994. Phytochrome-mediated responses: implications for controlled environment research facilities. (This proceedings)
Tibbitts, T.W., D.C. Morgan and I.J. Warrington. 1983. Growth of lettuce, spinach, mustard and wheat plants under four combinations of high-pressure sodium, metal halide, and tungsten halogen lamps at equal PPFD. J. Amer. Soc. Hort. Sci. 108:622-630.
Vince-Prue, D. 1975. Photoperiodism in plants. McGraw Hill, London, N.Y.
King, R.W., and D.J. Bagnall, 1994. Phytochrome, plant growth and flowering, p 103-109. In: T.W.Tibbitts (ed.). International Lighting in Controlled Environments Workshop, NASA-CP-95-3309.
Copyright © March 1994 NASA [National Aeronautics and Space Administration].
All rights reserved.