Light is undoubtedly the most important environmental variable for plant growth and development; plants not only use radiant energy in photosynthesis, they also respond to the quantity, quality, direction and timing of incident radiation through photomorphogenic responses that can have huge effects on the rate of growth and the pattern of development. It is surprising, therefore, that the manufacturers and suppliers of controlled environment facilities have been singularly uninventive in the design of the lighting assemblies they provide. The consumer has one choice only – a lighting assembly that provides irradiance levels usually only a fraction of sunlight, and a control system that is limited to regulating the timing of the on-off switch. The reasons for these limitations are partly technological, but in the main they result from ignorance on the part of both the consumer and the manufacturer. A specific and powerful example of this ignorance relates to the importance of the so-called far-red wavelengths (FR = 700-800 nm). Because the human eye can hardly detect wavelengths above 700 nm, and photosynthesis also cuts off at ca. 700 nm, the majority of plant and crop physiologists are still almost completely unaware that FR radiation can have massive effects on growth rate and development. In consequence, most growth cabinets have light sources based on fluorescent tubes, and provide very little FR apart from that emitted by a token number of small incandescent bulbs. Larger growth facilities often use broader spectrum light sources, but growth facilities that provide the capability to vary the FR incident upon the plants are about as abundant as seals in the Sahara. This article sets the background of the significance of FR radiation in the natural environment and its importance for plant growth and development in the hope that it might inform intelligently those concerned with improving the design of plant growth facilities.
The Natural Radiation Environment
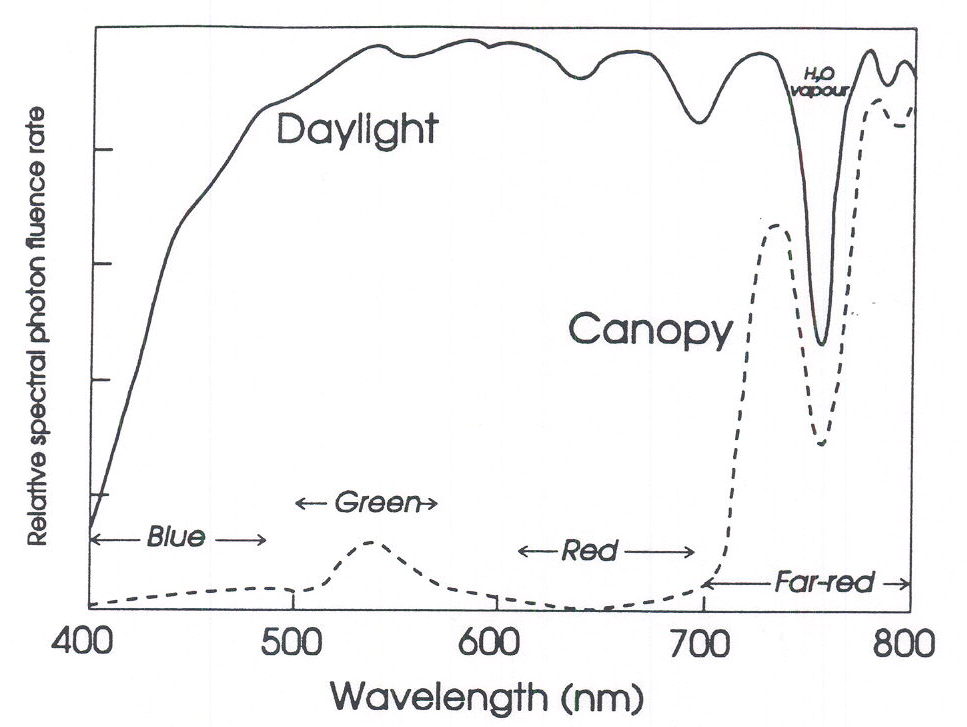
The daylight spectrum. The light environment experienced by plants in nature is obviously complex, but a number of generalisations can usefully be made. Solar radiation outside the atmosphere is distributed according to Planck’s radiation distribution law, with the sun behaving as a blackbody emitter with an apparent surface temperature approximating 5800o K. From Wien’s simplifications of Planck’s radiation formulae, the wavelength of maximum quantum emission is ca 620 nm, whereas in energy terms it is ca 500 nm; radiant emission falls off sharply at lower wavelengths and more gradually at higher wavelengths. This means that about 55% of the radiation incident on the earth’s surface falls within the 380-800 nm range of photochemical activity – which is fortunate, because photochemistry drives the energetic reactions of the biosphere via photosynthesis. Atmospheric components including ozone, oxygen, water vapour and carbon dioxide selectively absorb narrow wavelength bands, resulting in the typical radiation distribution of daylight at the earth’s surface seen in Figure 1. This radiation distribution is remarkably constant, being affected little by clouds and other climatic conditions (Holmes and Smith, 1977a). Pathlength through the atmosphere is important, of course, and as pathlength increases with the sun’s approach to the horizon at dusk (or dawn), refraction and Rayleigh scattering (inversely proportional to the fourth power of the wavelength) gives dawn/dusk radiation distributions with relatively elevated levels of blue light, and slightly increased levels of FR compared to daylight.
Fig. 1. The spectral distribution of daylight at the earth’s surface (solid line) and under a dense vegetation canopy (dotted lines).
Underwater light. The underwater light environment is of major importance, since more than half of plant life is underwater. Refraction at the air-water discontinuity leads to the incident light from above being concentrated into a cone of half-angle 48.6° ; consequently, a sensor facing upwards below, but near to the surface inevitably receives a proportion of upwelling radiation reflected back down from the surface. More important phenomena, as far as radiation distribution is concerned, are scattering and absorption by water itself, and by dissolved molecules or suspended particles. Rayleigh scattering results in the selective attenuation of the blue region of the spectrum of downwelling radiation. Water has strong absorption bands at ca. 730 nm and in the near infra-red, and therefore the FR is also selectively attenuated. Thus, in clear water, downwelling radiation is effectively “compressed” with increasing depth into a decreasingly narrow band of wavelengths, usually peaking at or around 500 nm. Absorption and scattering by algae, or by organic debris, causes the spectral distribution of radiation in turbid waters to be very variable.
The light environment within vegetation canopies. Ecologically, the most important fluctuations in radiation distribution occur when radiation interacts with vegetation. The photosynthetic pigments, the chlorophylls and carotenoids, absorb radiation over almost the whole of the visible spectrum (i.e. 400-700 nm). A small fraction of the “green” radiation is either transmitted or reflected, which is why leaves are green to our eyes. What is not so immediately obvious is that vegetation hardly absorbs any radiation between 700 and 800 nm. Thus, virtually all the incoming FR is either transmitted or reflected; i.e. the FR is scattered either through the leaf, or from the surface of the leaf. Since our visual systems are very insensitive to radiation beyond ca 700 nm, we fail to recognise that leaves should look far-red, rather than green! Figure 1 shows a typical daylight spectrum within a dense vegetation canopy, and demonstrates the marked depletion of red (i.e., R, 600-700 nm) and the relative enhancement of FR radiation within canopies.
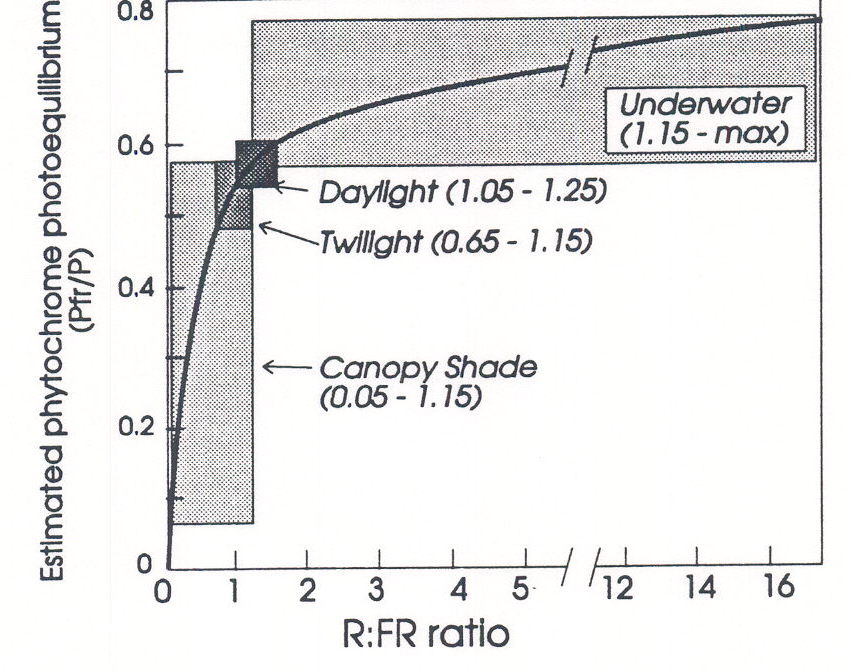
Fig. 2. The relationship between R:FR ratio and phytochrome photoequilibrium (Pfr/P). The shaded areas indicate the ranges of R:FR that are found under ecologically important conditions. Modified from Smith (1982).
The extent to which R is depleted and FR relatively enhanced by vegetation varies, of course, with the density of the canopy and the depth of the sensor within that canopy, and direct relationships with leaf area index have been established (Holmes and Smith, 1977b). A more subtle effect of vegetation on the relative amounts of R and FR radiation depends on the direction of propagation of the radiation being measured, or perceived. Unfiltered solar radiation is propagated downwards and is highly directional; i.e., only slightly scattered. After interaction with the leaves of a vegetation canopy, multiple scattering occurs, causing the radiation to be propagated more randomly. This means that radiation propagated more-or-less horizontally within a canopy will already have interacted with vegetation and will consequently be depleted in R and relatively enriched in FR, compared to radiation within a canopy that is propagated more-or-less vertically downwards (Smith, Casal and Jackson 1990). This point has a far-reaching significance, as will become evident later.
The biological significance of the variations in the relative amounts of R and FR radiation in the natural environment is that they provide signals of vital ecological importance. Plants have evolved a sophisticated battery of photoreceptors that enable them to sense environmental variations in R and FR and to use the information so obtained to direct appropriate alterations in metabolism, growth and development. Perception of environmental R and FR allows plants to detect the presence of neighbours, to gauge their competitive threat, and to react to actual or incipient shade by appropriate redirection of growth and development. The photoreceptors responsible for the perception of R and FR are the phytochromes.
The Phytochromes – Sensors of the Natural Radiation Environment
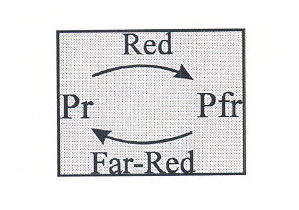
The Phytochrome family. The phytochromes are a family of photochromic photoreceptors each member of which consists of an apoprotein bearing a linear tetrapyrrole chromophore. Each phytochrome is capable of existing in two stable forms: Pr, which absorbs maximally at ca. 660 nm, and Pfr, which absorbs maximally at ca. 730 nm. Upon the absorption of radiation, Pr is photoconverted to Pfr, and Pfr photoconverted to Pr, according to the following scheme:
The absorption spectra of the Pr and Pfr forms of phytochrome isolated from etiolated oats show widely overlapping bands of absorption below ca. 730 nm, so that in broad-band radiation (such as daylight) both forms are continually photoexcited, resulting in a steady state photoequilibrium (defined as Pfr/P, where P = Pr+Pfr), in which the proportions of the total phytochrome present as Pr and Pfr are functions of the radiation distribution and of the absorption cross sections of Pr and Pfr. Since the absorption maxima are in the R and the FR, it is these wavelengths that are most important in achieving equilibrium. For this reason Smith and Holmes (1977) proposed that daylight spectra could be usefully characterised and simplified by measuring the ratio of radiation in two 10 nm wavebands centred on the absorption maxima of Pr and Pfr. Thus, the parameter R:FR, which is the ratio of the photon flux density in the 655-665 nm waveband, to that in the 725-735 nm waveband, has become the standard way of characterising daylight for photomorphogenic purposes.
There are known to be at least five members of the phytochrome family (i.e., phytochrome A to phytochrome E) in higher plants, as judged by Southern analysis of Arabidopsis genomic DNA (Sharrock and Quail 1989). Evidence from physiological studies of normal, mutant and transgenic phy gene overexpressers indicates that each member of the family probably has a distinct eco-physiological function, although functional overlap may occur under certain circumstances (Smith and Whitelam 1990). On this basis, the phytochromes represent a battery of photosensors that enable plants to obtain ecologically significant information from the light environment.
R:FR Ratio and Pfr/P. The benefit of using R:FR as a simplified parameter of the spectral distribution of natural radiation lies in the fact that R:FR can be readily transformed into a measure of the relative proportions of Pr and Pfr present at photoequilibrium. Figure 2 shows the hyperbolic relationship that exists between R:FR (as defined above) and Pfr/P (Smith and Holmes, 1977). Using this relationship, any measured value of R:FR can be transformed to a value which represents the Pfr/P to be expected in the outer epidermis of the irradiated tissue (ignoring any light reflected or scattered back from within that tissue); the transformation is made simply by reading Pfr/P from the curve for any measured value of R:FR. The actual direct measurement of Pfr/P in light-grown plants yet eludes the advance of analytical technology, mainly because there is very little phytochrome present and its absorption is overwhelmed by that of chlorophyll. The parameter arrived at by transforming R:FR is not necessarily an accurate measure of the real Pfr/P in the tissue; it is merely a physiologically-relevant way of expressing the relative amounts of R and FR in the incident radiation. Reading Pfr/P from the curve in Figure 2 is inadvisable for artificial sources in which either the R or FR is filtered out, or for sources in which blue light (which is capable of photoconverting Pr and Pfr) predominates. Under these circumstances it is advisable to integrate the spectral photon irradiances for the 400-800 nm waveband with the extinction coefficients of Pr and Pfr and the quantum efficiencies of the Pr Pfr and Pfr Pr phototransformations. In other words, the influence of light over the whole wavelength region absorbed by phytochrome on the photoconversion of Pr to Pfr, and vice versa, can be calculated, resulting in a more meaningful value for Pfr/P than can be derived from R:FR. Simple computer protocols exist for this transformation.
The relationship between R:FR and Pfr/P in Figure 2 reveals three important points. First, because R:FR during the day is very constant and unaffected by weather conditions, it provides the plant with a norm against which fluctuations in R:FR due to other environmental conditions may be compared. Secondly, underwater, R:FR increases very sharply with depth of immersion, but because the underwater values of R:FR are on the asymptote of the relationship between R:FR and Pfr/P, it is clear that phytochrome would be an insensitive detector of depth underwater. Thirdly, and most importantly, small reductions in R:FR caused by vegetation shade, or the proximity of neighbours, cause relatively large reductions in Pfr/P. Thus, because shade R:FR values lie on the steep part of the hyperbolic curve, the phytochromes in principle have the capacity to be very sensitive detectors of shade.
The Phytochrome-Mediated Shade Avoidance Syndrome
The nature of shade avoidance reactions. The acclimative responses of herbaceous plants to shade from other vegetation can be viewed in terms of two extreme strategies (Grime 1979). One strategy, that of shade tolerance, involves relatively slow growth rates, the conservation of energy and resources, perennation usually by vegetative processes, and the development of photosynthetic structures that are especially efficient at low light levels. The opposite extreme is shade avoidance, a syndrome of growth and developmental changes in which extension growth is favoured at the expense of leaf and storage organ development. As the name suggests, if successful, shade avoidance has the overall effect of projecting the photosynthetic structures (usually leaves) into those parts of the environmental mosaic in which the resource of light is plentiful. Shade avoiders tend to be photosynthetically inefficient at low light levels, but have the capacity rapidly to direct growth potential from leaf development to shoot extension upon the first detection of incipient shading. Shade avoidance is an effective strategy for life in an herbaceous community, but has limitations for herbs growing on the floor of a dense forest. The two strategies, avoidance and tolerance, are not necessarily mutually exclusive, since some plants display intermediate strategies and appear to be able to adapt to life either in open or shaded habitats, whilst other plants can exhibit shade avoidance and shade tolerance at different points in their life cycle.
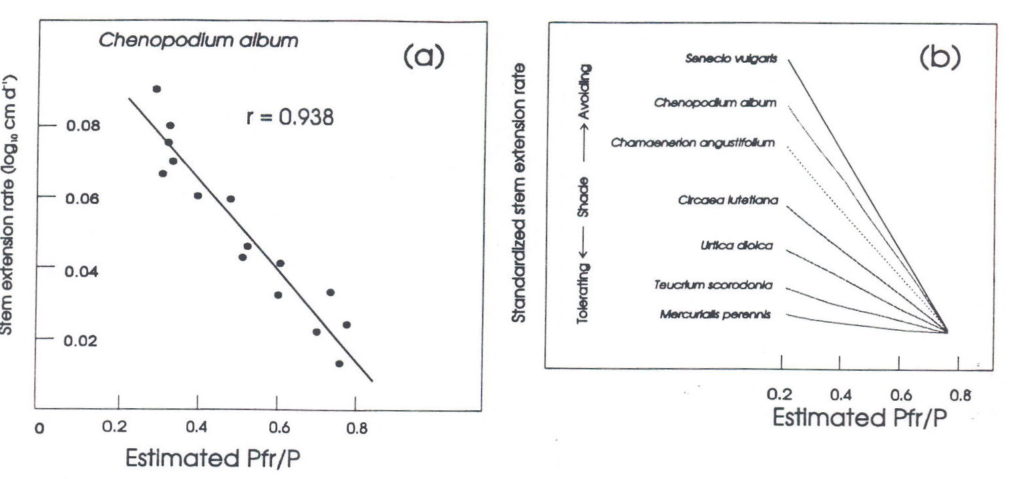
Phytochrome-mediation of shade avoidance. When shade avoiding species are grown in white light to which various amounts of FR have been added, developmental responses essentially similar to those seen in natural canopy shade result (Morgan and Smith 1978, 1979; Smith 1982; Smith and Morgan, 1983; Casal and Smith 1989). Figure 3a shows the relationship between extension growth and the predicted Pfr/P for seedlings of the shade avoiding weed Chenopodium album grown in cabinets in which the PAR was held uniform but the R:FR was decreased by supplementation with varying flux densities of FR. This figure presents the most striking effect of supplemental FR, which is the enhanced elongation growth at low R:FR, but all the other components of the shade avoidance syndrome can be induced by such decreases in R:FR. For example, growth in WL+FR causes a major redistribution of assimilates from leaf expansion and storage organ accumulation to stem and petiole growth (Keiller and Smith 1988). It also strongly accelerates flowering (Robson, Whitelam and Smith, 1993), a phenomenon as yet little studied but potentially of considerable importance.
Thus, the induction of the shade avoidance syndrome requires the perception of the spectral changes associated with shade, rather than the changes in total light quantity. Furthermore, the linear relationship between extension rate and calculated phytochrome photoequilibrium (Figure 3a) has been shown to obtain for a wide range of species (Morgan and Smith 1979), providing convincing evidence that the perception of shade and the induction of shade avoidance responses is phytochrome-mediated. In particular, the magnitude of extension growth responses to added FR is related to the life style of the plant; i.e., shade avoiders respond strongly, whilst shade tolerators respond weakly (Figure 3b) (Morgan and Smith 1979).
Two further important characteristics of R:FR perception are its rapidity and its compensation for changes in irradiance. Using position-sensitive transducers to enable the continuous monitoring of stem extension rate, changes in extension rate caused by FR radiation applied to the growing internode via fibre-optic probes can be detected within minutes (Morgan, O’Brien and Smith 1980; Child and Smith, 1987). Furthermore, within wide limits, the extension rate is determined by the R:FR at the internode and is independent of the flux density of white light presented from above (Child and Smith 1987). These results indicate that the perception of R:FR is precisely quantitative and is compensated for variations in total irradiance. This means that, in principle, phytochrome-mediated R:FR perception should not only be able to operate at the light levels that exist within dense canopies, but should also function at the high irradiances present in sparse stands of plants that are not sufficiently close to cast actual shade. That R:FR perception does indeed occur at very high flux densitys was shown by Smith (1990) who observed strong accelerations of extension rate in mustard seedlings when exposed to high levels of FR added to a background white light in excess of 1500 µmol m-2 s-1.
Fig. 3. The linear relationship between Pfr/P (estimated from the incident radiation spectrum) and the rate of stem extension growth. (a), data for Chenopodium album; (b), normalised data from a range of shade-avoiding and shade-tolerating plants. Modified from Smith (1982).
Neighbour detection and proximity perception. This latter point leads to the expectation that plants should be able to detect the FR reflected from neighbours before actual shading occurs, thereby providing for anticipation of competition for light. That this occurs in nature was suggested by Kasperbauer et al. (1984) in studies of soybeans grown in either north-south or east-west rows. R:FR near the top of the north-south rows of plants was lower on the west side in the morning, and on the east in the evening; indicating that the adjacent rows act as FR reflectors when the sun is low in the sky. The fact that mustard plants growing under background white light in growth cabinets react very rapidly to FR directed horizontally at the growing internodes, also indicates that plants can detect horizontally-propagated FR whilst being exposed to high R:FR light from above. Ballaré et al. (1987, 1990) have demonstrated direct effects of reflected FR on plant growth in the field using seedlings of Datura ferox, a strongly shade-avoiding herb, grown in the field close to grass screens that were either green, or bleached by being sprayed with a herbicide. The plants adjacent to unbleached, green hedges grew significantly faster than those near to the bleached hedges. Datura ferox seedlings, when inserted into a sparse canopy of similar seedlings not dense enough to cast actual shade, grew faster than in the open. If their growing internodes were surrounded by transparent collars containing dilute copper sulphate solution (which absorbs FR), there was no increase in growth. Thus, phytochrome-mediated R:FR perception is sufficiently sensitive to allow the detection of reflected light from neighbouring vegetation. Smith et al. (1990) measured the reflection signals from stands of tobacco, and also measured actual Pfr/P in samples of purified oat phytochrome exposed to the radiation reflected from the tobacco stands. These data showed that, in principle, neighbour detection could operate over substantial distances, and that the signals progressively increased with proximity to the neighbours. In nature, therefore, plants are able to detect the FR reflected from neighbours even though the FR flux is a tiny fraction of that of the vertically propagated, high R:FR daylight. It seems that simple geometry may be responsible for this apparent paradox, as the most sensitive regions are the growing internodes which, for most shade avoiding species, are held erect, thereby receiving very little downwardly propagated radiation. On this basis, phytochrome-mediated R:FR perception provides plants with the capacity for proximity perception; in other words, plants not only can detect their neighbours, they can effectively perceive how far away they are, and therefore are able to gauge the competitive threat posed.
In summary, plants are exceptionally sensitive to the relative amounts of R and FR they receive. In nature, the shade avoidance syndrome provides plants with the capacity to adapt rapidly to the competitive threat posed by neighbours. In the controlled environment, manipulation of the R:FR ratio can give the experimenter, or the grower, impressive control over the pattern of development and the rate of growth.
Implications for the Design of Plant Growth Facilities
There are, of course, alternative ways of varying R:FR in controlled environments; one can add FR to a constant R, one can add R to a constant FR, or one can vary both R and FR simultaneously. The latter is what happens in the natural environment, but in our hands the objective has been to dissociate the phytochrome-mediated responses to varying R:FR, from any effects that might be a result of changes in photosynthetic rates caused by reduced levels of photosynthetically active radiation. Ideally, we would like to have growth facilities in which photosynthetic rate could be held uniform at high levels, whilst simultaneously varying the proportions of Pr and Pfr; for this reason we have concentrated on designing cabinets that provide a constant, uniform background of white light (and therefore constant R) whilst varying R:FR by adding FR. This approach inevitably carries a number of technical problems.
At present, no sources are available at an affordable cost that provide high irradiance FR without also emitting large amounts of longer wavelength infra red. The simplest way of producing FR is to filter the radiation emitted by incandescent sources, but these have maximum emissions at ca 900 nm or higher, and put out a great deal of radiation in the longer wave infra red. Consequently, using such sources inevitably means that one has to remove a large amount of radiant heat. Because of the technical problem of dealing with this heat, until recently we have been forced to use cabinets in which the background WL was of relatively low irradiance. In our latest designs, we have developed cabinets in which high irradiance broad-band WL can be supplied from above, with high irradiance FR being provided horizontally from sources mounted in the side walls; these cabinets were built as a direct response to the realisation of the importance of horizontally-propagated radiation.
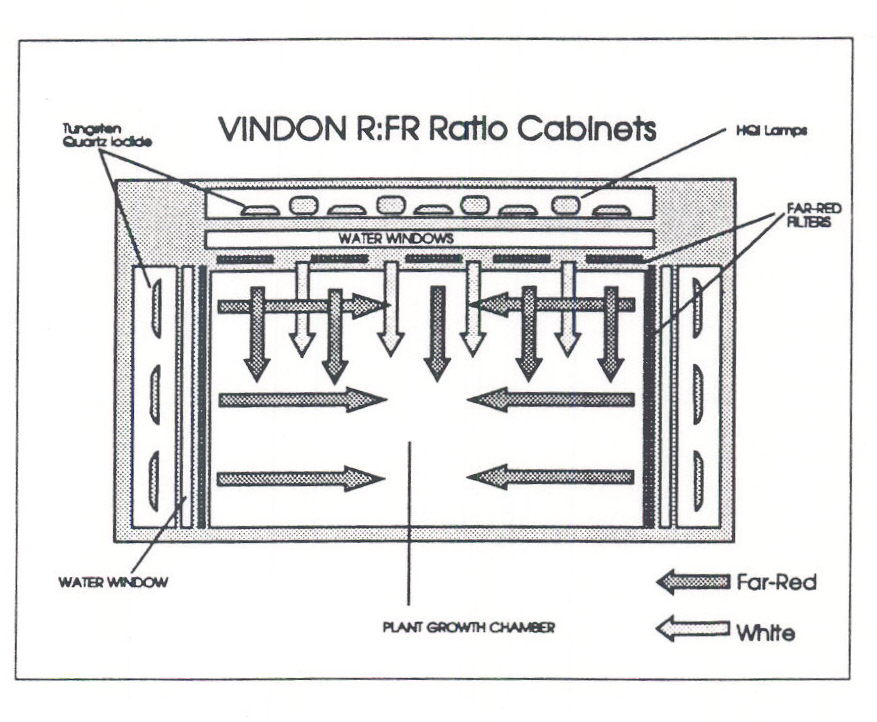
Removal of radiant heat can only be achieved by absorption of the heat, followed by some form of heat exchange. Our approach has been to use so-called “water windows”, in which flowing water, cooled by passage through a heat exchanger, absorbs the radiant heat emitted by the incandescent lamps used to provide the FR. Following radiant heat absorption, the FR is selected by the use of Perspex (Plexiglass) filters. The design and construction of effective water-windows is by no means a trivial exercise, and when a window fails, the close proximity of gallons of water to high voltage circuitry can yield impressive pyrotechnic displays. Nevertheless, with appropriate fail-safe devices and controls, water windows can be reliable and safe, although the cost would be prohibitive for standard growth cabinets. With this approach we have been able to develop cabinets in which upwards of 500 mol m-2 s-1 FR can be added to background WL of between 150 mol m-2 s-1 PAR (old designs) and 400 mol m-2 s-1 PAR (new design). In the latest side-wall FR cabinets, we can provide 500 mol m-2 s-1 FR from the side and up to 900 mol m-2 s-1 PAR from above. The latest cabinets were designed by us and constructed to an extremely high degree of excellence by Vindon Scientific Ltd., based near Oldham in the UK. Figure 4 shows the essential features of the latest set of Vindon cabinets, and the legend includes the contact person and the address of the company.
Fig. 4. A schematic diagram of a plant growth cabinet designed to provide high flux density white light from above which can be supplemented with high flux density FR radiation either from above, or from the sides. Removal of the FR filters from the upper lighting compartment provides a white light spectrum that simulates daylight reasonably well, provides a flux density of 900 mmol m-2 s-1 at 1 m from the compartment window, and has a R:FR ratio of ca 1.5. Radiant heat is removed by ‘water windows’, containing running water cooled by external refrigerating heat exchangers. The flux density of FR that may be supplied, either from above or the sides, is ca 500 mmol m-2 s-1. This cabinet allows the possibility to grow plants in high flux density white light from above but to establish varying phytochrome photoequilibria by supplementation with FR from the sides. (Further details of these cabinets may be obtained from the author, or from the manufacturer, by contacting Mr Alan Roylance, Vindon Scientific Ltd., Diggle, nr Oldham, Lancashire, UK).
Bringing water and electricity close together should, of course, be avoided. If other sources of high irradiance FR were to become available (i.e., discharge lamps, LEDs, micro-wave-driven sources, etc.) at a reasonable cost, then the design of cabinets that allow the simulation of natural R:FR ratios whilst simultaneously providing satisfactory photosynthetic rates would be simplified, and a major improvement in growth cabinet design could be contemplated. Both growers and experimenters would then be able to achieve much better control of the growth and development of their plants.
REFERENCES
Ballaré, C. L., Sânchez, R. A., Scopel, A. L., and Ghersa, C. M. (1987). Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant, Cell Environ. 10, 551-557
Ballaré, C. L., Scopel, A. L., and Sânchez, R. A. (1990). Far-red radiation reflected from adjacent leaves: An early signal of competition in plant canopies. Science 247, 329-332
Casal, J.J. and Smith, H. (1989) The function, action and adaptive significance of phytochrome in light-grown plants. Plant, Cell and Environment 12, 855-862
Child, R. and Smith, H. (1987) Phytochrome action in light-grown mustard: Kinetics, fluence-rate compensation and ecological significance. Planta 172: 219-229.
Grime, J. P., 1979. Plant Strategies and Vegetation Processes. John Wiley, London.
Holmes, M.G. and Smith, H. (1977a) The function of phytochrome in the natural environment. I. Characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochem. Photobiol. 25, 533-538.
Holmes, M.G. and Smith, H. (1977b) The function of phytochrome in the natural environment. II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochem. Photobiol. 25, 539-545.
Kasperbauer, M. J., Hunt, P. G., and Sojka, R. E. (1984). Photosynthate partitioning and nodule formation in soybean plants that received red or far-red light at the end of the photosynthatic period. Physiol. Plant. 74, 415-417
Keiller, D., and Smith, H. (1989) Control of carbon partitioning by light quality mediated by phytochrome. Plant Science 63: 25-29
Morgan, D.C., O’Brien, T. and Smith, H. (1980) Rapid photomodulation of stem extension in light-grown Sinapis alba L. Studies on kinetics, site of perception and photoreceptor. Planta 150, 95-101.
Morgan, D.C. and Smith, H. (1978) The function of phytochrome in the natural environment. VII. The relationship between phytochrome photo-equilibrium and development in light-grown Chenopodium album L. Planta 142, 187-193.
Morgan, D.C. and Smith, H. (1979) A systematic relationship between phytochrome-controlled development and species habitat for plants grown in simulated natural radiation. Planta 145, 253-259
Robson, P. R. H., Whitelam, G. C., and Smith, H. (1993) Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 102, 1179-1184.
Sharrock, R. A. and P. H. Quail (1989) Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Devel. 3, 534-544.
Smith, H. and Holmes, M.G. (1977) The function of phytochrome in the natural environment. III. Measurement and calculation of phytochrome photoequilibrium. Photochem. Photobiol. 25, 547-550.
Smith, H., 1982 Light quality, photoperception and plant strategy. Ann. Rev. Pl. Physiol. 33, 481-518.
Smith, H., and Morgan, D. C., (1983). The function of phytochrome in nature, In: Encyclopedia of Plant Physiology, New series, 16B, Photomorphogeniesis, Shropshire, Jr., W., and Mohr, H. eds., pp. 401-517, Soringer-Verlag, Berlin.
Smith, H., and Whitelam, G.C. (1990) Phytochrome, a family of photoreceptors with multiple physiological roles. Plant, Cell and Environment 13, 695-707.
Smith, H., Casal, J.J. and Jackson G.M. (1990) Reflection signals and the perception by phytochrome of the proximity of neighbouring vegetation. Plant, Cell and Environment 13: 73-78.
Smith, H. 1994. Phytochrome-mediated responses – Implications for controlled environment research facilities, p 57-67. In: T.W.Tibbitts (ed.). International Lighting in Controlled Environments Workshop, NASA-CP-95-3309.
Copyright © March 1994 NASA [National Aeronautics and Space Administration].
All rights reserved.